Pilot work of medical device unique identification system
For the implementation of the general office of the central committee of the general office of the State Council, on deepening the reform of the review and approval system encourages drugs and medical equipment innovation and the State Council General Office of the key tasks of deepening the reform of the medical and health system in 2019, the national drug administration jointly with the national health commission to carry out the medical equipment only identification system pilot work.
In order to thoroughly implement the decisions and arrangements on implementing the Healthy China strategy and deepening the reform of the medical and health care system, To implement Xi Jinping's "four strictest" requirements on drug safety, Strengthen the supervision of the whole life cycle of medical devices, Preliminary establishment of a medical device unique identification system, To realize the demonstration and application of unique identification in production, operation, circulation and use. Explore the formation of the whole chain linkage from the source of production to the final clinical use, Accumulate the experience continuously, In order to fully implement the unique medical device identification system in the later stage, To improve the efficiency of medical device supervision and health management, To effectively ensure the safety of the public for equipment use, Lay the foundation for promoting the formation of a new pattern of supervision and governance of medical devices.
CFDA Medical Device Unique Identification System Rules
Article 1 These Rules are formulated in accordance with the Regulations on the Supervision and Administration of Medical Devices for the purpose of standardizing the construction of the unique identification system of medical devices and strengthening the whole life cycle management of medical devices.
Article 2 The unique identification system of medical devices sold or used in the territory of the People's Republic of China shall comply with these Rules.
Article 3 The unique identification system of medical devices as mentioned in these Rules is composed of unique identification of medical devices, unique identification data carrier and unique identification database.
The unique identification of medical devices refers to the code composed of numbers, letters or symbols attached to the medical device product or package for the unique identification of medical devices.
The unique identification data carrier of medical device refers to the data media that stores or transmits the unique identification of the medical device.
The unique identification database of the medical device refers to the database storing the product identification and associated information of the unique identification of the medical device.
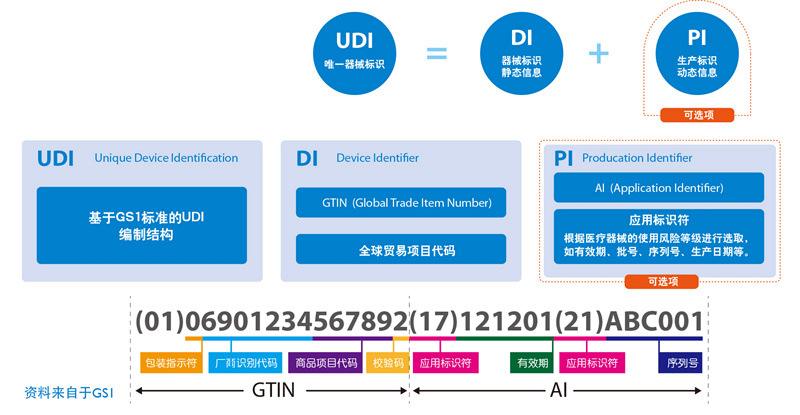
Article 7 The unique identification of medical devices includes product identification and production identification.The product identification is the unique code to identify the registrant / applicant, medical device model specification and packaging; the production identification consists of the production process information of the medical device, which can include the medical device serial number, production batch number, production date, failure date, etc. according to supervision and actual application requirements.
New product labels shall be created in case of changes that may affect the identification or traceability of medical devices, or changes in regulatory requirements.
If the medical devices are not sold and used, the product identification shall not be used for other medical devices; the original product identification may be used.
Article 8 The unique identification of a medical device shall meet the requirements of uniqueness, stability and scalability.
Uniquity means that the unique identification of a medical device should be consistent with the medical device identification requirements.
Stability means that the unique identification of a medical device shall be related to the basic characteristics of the product, and if the basic characteristics of the product have not changed, the product identification shall remain unchanged.
Scalability means that the unique identification of medical devices should adapt to the continuous development of regulatory requirements and practical applications.
Article 11 The unique identification data carrier of a medical device shall meet the requirements of automatic identification and data collection technology and manual reading.If space is limited or use is limited, priority should be given to automatic identification and data acquisition technology.
Automatic identification and data acquisition technologies include one-dimensional code, QR code or radio frequency label, encouraging the adoption of advanced automatic identification and data acquisition technologies.
When one-dimensional code is used, product identification and production identification can be connected in series or in parallel, one-dimensional code or QR code should be used.
Article 13 The State Medical Products Administration shall formulate standards and norms related to the unique identification data of medical devices, and organize the establishment of the unique identification database of medical devices for public inquiry.
Article 17 The meanings of the following terms used in these Rules:
Automatic identification and data acquisition refers to the technology that does not directly input the data into the computer system or other microprocessor-controlled equipment through the keyboard.
Manual reading refers to the coding information corresponding to the machine reading media and which can be directly recognized by the human eye.
Article 18 These Rules shall come into force as of October 1,2019.The specific steps for classified implementation shall be formulated and published separately.
State Food and Drug Administration
(Excerpts for details at the end of the article)
UDI marking solution
Unique Device Identification(UDI) is an "identification system for special medical devices" established by the FDA in the United States. It is the identity identification given to the medical device in its entire life cycle, and it is the only "ID card" in the product supply chain.It is mainly a code composed of numbers or letters.It consists of device identification code (DI) and production identification code (PI).

Dikai products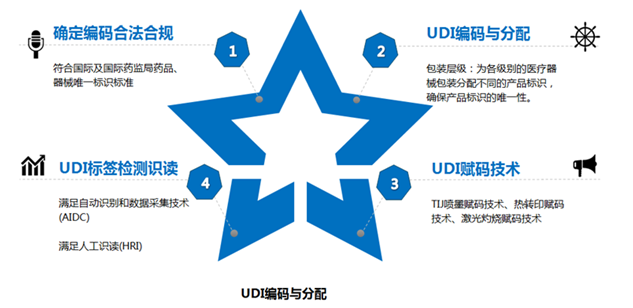
Dikai products
According to the UDI coding standard of medical devices, make the code according to different materials, such as: box packing, metal materials, all kinds of equipment packaging materials, soft packaging, etc.Can choose hot transfer printing machine(TTO), laser marking machine, inkjet printer and other products.
Suitable for code content: bar code, one-dimensional code, two-dimensional code, number, text, English, etc.Code content is clear, high read rate.





Scan QR code to subscribe DK public account 
系统功能强大、高效、高速、稳定